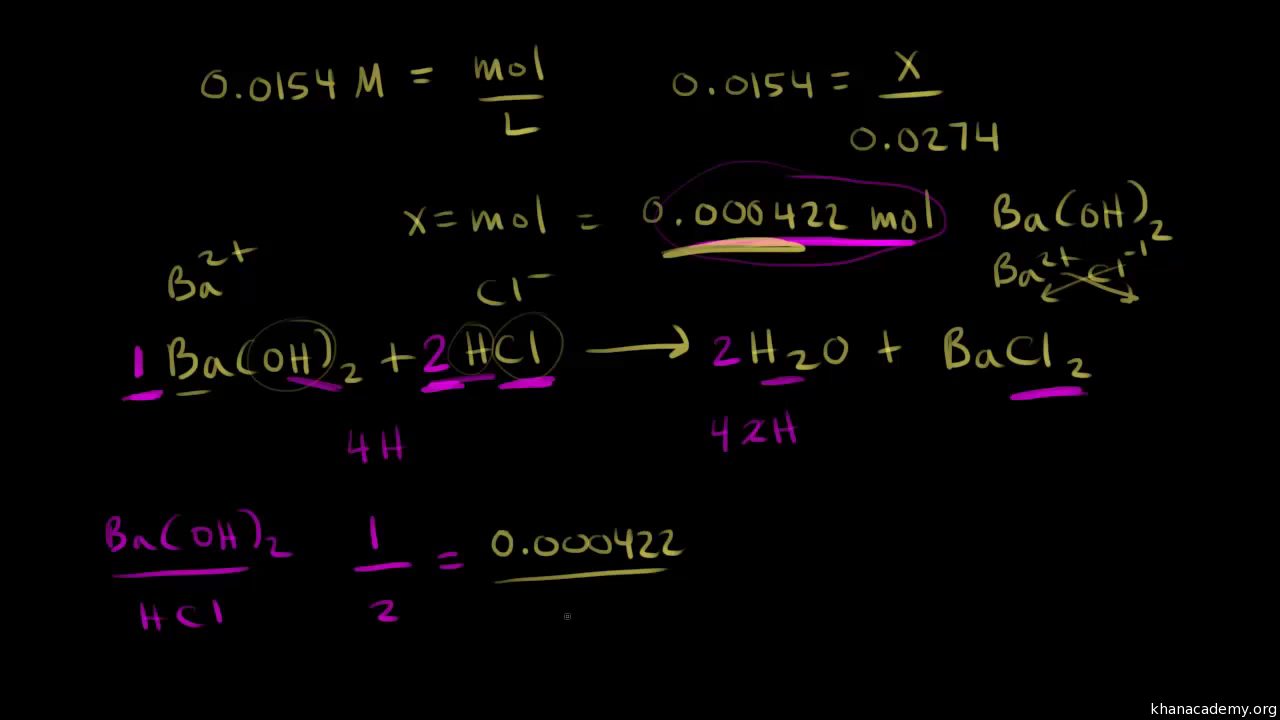How To Calculate Titration Problems . Calculating ph for titration solutions: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. What was the concentration of the hcl? You will also learn about equivalence points and. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is added to a solution of another substance to determine its concentration. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl.
from tukioka-clinic.com
One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is added to a solution of another substance to determine its concentration. Calculating ph for titration solutions: You will also learn about equivalence points and. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. What was the concentration of the hcl? A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). First determine the moles of \(\ce{naoh}\) in the reaction.
👍 Solve titration problems. How to solve titration problems step by
How To Calculate Titration Problems A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Calculating ph for titration solutions: In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn about equivalence points and. What was the concentration of the hcl? One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is added to a solution of another substance to determine its concentration. First determine the moles of \(\ce{naoh}\) in the reaction. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog How To Calculate Titration Problems A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is added to a. How To Calculate Titration Problems.
From fity.club
Titration Calculations How To Calculate Titration Problems From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. First determine the moles of \(\ce{naoh}\) in the reaction. You will also learn about equivalence points and. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample. How To Calculate Titration Problems.
From fity.club
Titration Formula How To Calculate Titration Problems In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. Calculating ph for titration solutions: What was the concentration of the hcl? From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted.. How To Calculate Titration Problems.
From www.tffn.net
Solving Titration Problems A StepbyStep Guide The Enlightened Mindset How To Calculate Titration Problems Calculating ph for titration solutions: One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is added to a solution of another substance to determine its concentration. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with.. How To Calculate Titration Problems.
From annbjordanxo.blob.core.windows.net
Titration Chemistry Practice Problems How To Calculate Titration Problems In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn about equivalence points and. Calculating ph for titration solutions: First determine the moles of \(\ce{naoh}\) in the reaction. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. From the mole ratio, calculate. How To Calculate Titration Problems.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube How To Calculate Titration Problems You will also learn about equivalence points and. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. First determine the moles of \(\ce{naoh}\) in the reaction. Calculating ph for titration solutions: In. How To Calculate Titration Problems.
From www.youtube.com
Back Titration Example YouTube How To Calculate Titration Problems First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. What was the concentration of the hcl? One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is added to a solution of another substance to determine its concentration. Calculating ph. How To Calculate Titration Problems.
From www.nagwa.com
Question Video Calculating the Mass of Solute in a Solution via How To Calculate Titration Problems What was the concentration of the hcl? First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is added to a solution of another substance to determine its concentration. In this. How To Calculate Titration Problems.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration How To Calculate Titration Problems A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. What was the concentration of the hcl? Calculating ph for titration solutions: One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is added to a solution of another substance to determine. How To Calculate Titration Problems.
From www.youtube.com
Solving AcidBase Titration Problems YouTube How To Calculate Titration Problems Calculating ph for titration solutions: You will also learn about equivalence points and. One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is added to a solution of another substance to determine its concentration. In this tutorial, you will learn about titration curves, titration analysis and the steps required to. How To Calculate Titration Problems.
From fity.club
Titration Calculations How To Calculate Titration Problems A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. One such method is a titration, in which a measured volume of a solution of. How To Calculate Titration Problems.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions How To Calculate Titration Problems What was the concentration of the hcl? Calculating ph for titration solutions: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. You will also learn about equivalence points and. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. First determine the moles of \(\ce{naoh}\). How To Calculate Titration Problems.
From fity.club
Titration Curve How To Calculate Titration Problems You will also learn about equivalence points and. First determine the moles of \(\ce{naoh}\) in the reaction. Calculating ph for titration solutions: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A 25 ml solution of 0.5 m naoh is titrated until neutralized. How To Calculate Titration Problems.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube How To Calculate Titration Problems A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). First determine the moles of \(\ce{naoh}\) in the reaction. You will also learn about equivalence points and. Calculating ph. How To Calculate Titration Problems.
From www.chegg.com
Solved To review how titration calculations work, here's a How To Calculate Titration Problems You will also learn about equivalence points and. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. What was the concentration of the hcl? Calculating ph for titration solutions: First determine the moles of \(\ce{naoh}\) in the reaction. In this tutorial, you will learn about titration curves, titration analysis and the steps required to. How To Calculate Titration Problems.
From www.youtube.com
Titration Calculations YouTube How To Calculate Titration Problems One such method is a titration, in which a measured volume of a solution of one substance, the titrant, is added to a solution of another substance to determine its concentration. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. In this tutorial, you will learn about titration curves, titration. How To Calculate Titration Problems.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube How To Calculate Titration Problems First determine the moles of \(\ce{naoh}\) in the reaction. What was the concentration of the hcl? A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. One such method is a titration, in which a measured volume of a solution. How To Calculate Titration Problems.
From www.youtube.com
Titration (sample problems) YouTube How To Calculate Titration Problems A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. You will also learn about equivalence points and. Calculating ph for titration solutions: In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). One such method is a titration, in which a measured. How To Calculate Titration Problems.